Haemair Granted Patent on Novel Pulsatile Blood Pump
November 17, 2022 2:28 pmThe Haemair pulsatile blood pump patent awarded in Europe in October 2021 is now being approved in other territories. The figure below shows a pump on test in our laboratory.

As well as the benefits of the unique pulsing flow that maintains the function of organs and tissues perfused, the device has the benefit of extremely low haemolysis. Low haemolysis corresponds to very little damage to red blood cells as blood is pumped through the device. Haemolysis has two deleterious impacts. The first is that it removes functioning red blood cells from the circulating blood. The second is that, in rupturing, the cells release toxic species into the blood including potassium ions, free iron, and free haem.
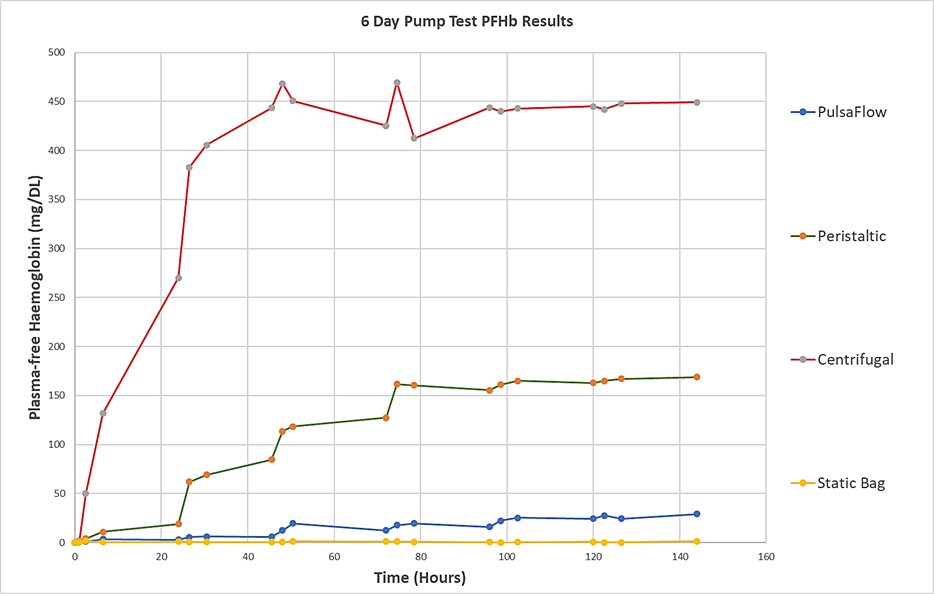
The figure below compares haemolysis caused by the Haemair pump with that caused by leading commercial centrifugal and peristaltic pumps. The graph compares the performance of the three pumps, using the same batch of blood, operating at exactly the same flow rate over 6 days. The graph shows that, at the low flow rates required for organ perfusion, a respected widely used centrifugal blood pump causes 15 times as much damage is the Haemair pump.
All mammals have beating hearts and all the organs and tissues expect pulsing flows. Without a pulsing flow, capillary blood vessels tend to close up with adverse impacts on the ability of the organs to continue to function. The pump has a range of potentially life-saving applications including:
- Ex-vivo perfusion of organs for transplant. Organs can be kept in a healthier state between harvesting and transplant by perfusing them with warm blood. A pulsing flow keeps these isolated organs alive longer giving more time to assess them. In this way, more transplant organs are made available, fewer retransplants needed, and waiting lists can be reduced.
- Cardiopulmonary bypass. In open-heart surgery, blood circulation is taken over by a blood pump. A pulsing flow should eliminate some of the problems currently found with patients perfused with a steady blood flow.
- Pharmaceutical testing. Currently millions of animals are used in drug testing. Many of the drugs tested are focused on diseases of specific organs and tissues. A pulsing blood flow that maintained extended ex-vivo viability of such organs and tissues would enable drugs to be tested in a much more focused and controlled way than in whole animal testing. At the same time the number of animals used for testing can be greatly reduced.
